Endoscopes help medical procedures to be less invasive, thereby reducing the risk of complications as well as costs and recovery times, but their application is limited in part by their size and inflexibility and by their inability to provide a three-dimensional perspective. We study a new type of endoscopy that enables video-rate, three-dimensional images to be transmitted from flexible probes that are comparable in diameter to a human hair. This technology opens up the possibility of moving
operations to an outpatient setting, reducing requirements for anesthesia, and minimizing tissue damage.
The first endoscope was invented almost 50 years ago and consisted of a bundle of optical fibers. Miniature endoscopes still use bundles of optical fibers to transmit a two-dimensional image, but larger endoscopes now employ solid-state, charge-coupled-device cameras for superior image quality. Fiber-bundle endoscopes with sub millimeter diameters have been used for a variety of clinical applications. However, they have not been widely adopted owing to their rigidity and inadequate image quality, which is due in part to relatively low numbers of pixels and superimposition of a honeycomb pattern (a pixelation artefact).
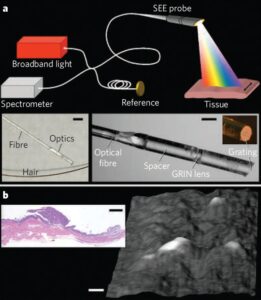
Spectrally encoded endoscopy (SEE) is a miniature-endoscopy technique that overcomes many of the limitations of fiber-optic imaging bundles. With SEE, polychromatic light emanating from a single optical fiber is configured such that each color (wavelength) is projected to a different location on the tissue surface (Fig. 1a). Light reflected from the patient can then be decoded outside the body by using a spectrometer, to form one line of the endoscopic image. The second dimension is obtained by moving the fiber using a mechanical-transduction mechanism, such as an external motor or a galvanometer. The diameter of the SEE probe can be as small as that of the optical fiber, which is typically in the range 80–250 μm. Even though the probe diameter is small, the number of pixels in a SEE image is larger than that from fiber bundles, being dependent only on the spectral width of the light source and the ability of the probe to separate out the components of different wavelengths. Spectral encoding can also provide depth information by using optical interferometry. Photographs of a prototype miniature SEE probe are shown in the insets of Fig. 1a. Light from a single-mode fiber, expanded through a 1.8-mm-long silica spacer, is focused by a gradient-index (GRIN) lens and then diffracted by a transmission grating (1,000 lines per mm) fabricated on the tip of the probe at Littrow’s angle. The maximum diameter of the probe is 350 μm. In combination with a broad-bandwidth (700–900 nm) titanium–sapphire laser, Michelson interferometer and high-speed spectrometer (see supplementary information), this miniature probe can be used to obtain volumetric images with about 400,000 resolvable points at video rates (30 frames per second).
To demonstrate the potential of SEE for minimally invasive applications, we imaged
metastatic ovarian tumour nodules on the peritoneum of a living mouse. The SEE probe was delivered through a modified 23-gauge needle into the abdominal cavity. A three-dimensional surface image of the parietal peritoneal wall, obtained with the SEE probe in vivo, is shown in Fig. 1b: several raised tumor nodules are evident and the inset shows a histological section.
The size and flexibility of this device will allow safer navigation through delicate intraluminal structures such as the fallopian tube, the salivary, mammary and pancreatic ducts, and safer fetal, pediatric and neurosurgical interventions. Navigation mechanisms are still needed that add minimal bulk without compromising flexibility. Color could be introduced by using three separate broad bandwidth sources, each centered at visible red, green or blue wavelengths. SEE using fluorescent light should also be useful for imaging labeled molecular species, reporters and molecular beacons.
References
- Three-dimensional miniature endoscopy
D. Yelin, I. Rizvi, W. M. White, J. T. Motz, T. Hasan, B. E. Bouma, and G. J. Tearney
Nature 443, 765(2006) - Miniature forward-viewing spectrally encoded endoscopic probe
Adel Zeidan and Dvir Yelin
Optics Letter 39, 4871 (2014)